Taking out heat
Just a few minutes after you put warm food in a refrigerator, the food
feels cooler. But the air inside the refrigerator doesn’t feel warmer.
What happens to the heat?
The heat doesn’t disappear. The refrigerator carries it from the food to
the room outside.
Many years before refrigerators were invented, scientists studied
liquids and gases. They found that when a liquid evaporates (ih
[vap]{.smallcaps} uh rayts), or changes to a gas, its molecules take
heat from the things around it. They also discovered that when a gas
condenses (kuhn [dehn]{.smallcaps} suhz), or changes to a liquid, its
molecules give o/fheat.
These discoveries made it possible for people to build refrigerators.
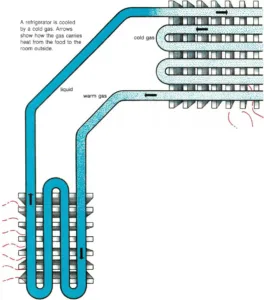
Refrigerators are cooled by a special liquid that is easily changed to a
gas and then back to a liquid again.
First, the cool liquid is pumped to tubes inside the refrigerator, where
it evaporates. As the liquid changes to a gas, its molecules take heat
from the air inside the refrigerator. This makes the refrigerator
cooler.
Then the warm gas is pumped to tubes outside the refrigerator, where it
condenses. As the gas changes back to a liquid, it gives off heat to the
air in the room.
When the liquid cools, it is pumped back into the refrigerator. There it
evaporates again. In and out it goes, carrying heat from the
refrigerator and keeping the food cold.