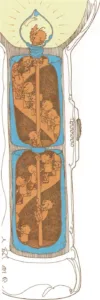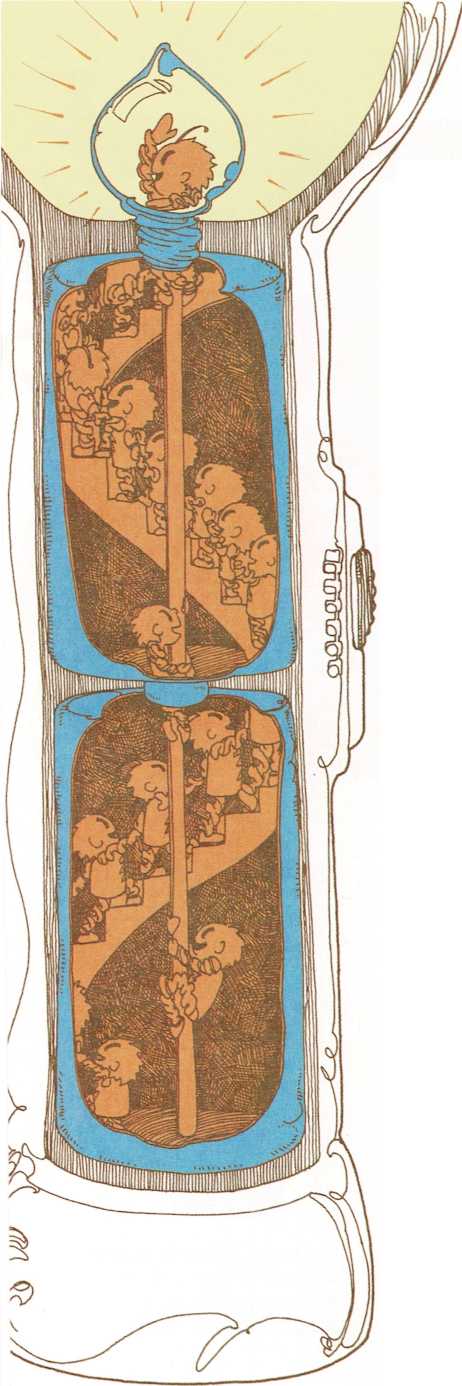
Packages of electricity
A flashlight runs on electricity—but you don’t have to plug it in. It
carries its electric current in a \”package”—a battery.
A battery is made of layers of chemicals inside a metal can. When the
flashlight is turned on, some of the chemicals in the battery break
apart and eat away at the metal can. As this happens, some of the metal
atoms leave the can and combine with the chemicals in the battery.
When the metal atoms move away from the can, they leave some of their
electrons behind. So the can gains electrons. And as the chemicals
inside the battery break apart, they lose electrons.
Soon, there are more electrons on the can than there are inside the
battery. Then the extra electrons on the can begin to move out of the
battery. They travel through the bulb and back into the middle of the
battery, where electrons are scarce. The push of these electrons is the
current that makes your flashlight shine.
This may make it seem that everything happens very slowly. But, as you
know, it all takes place in an instant.