As warm as toast
If you spread cold butter on hot toast, the butter won’t stay cold very
long. Some of the heat from the toast passes into it. Soon the butter is
warm, too—as warm as toast.
Heat energy can spread. It can be conducted (kuhn [duhk]{.smallcaps}
tuhd), or carried, from something warmer to something cooler. The
movement of molecules passes the heat along.
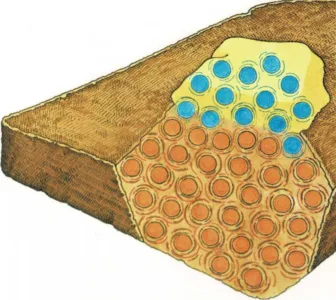
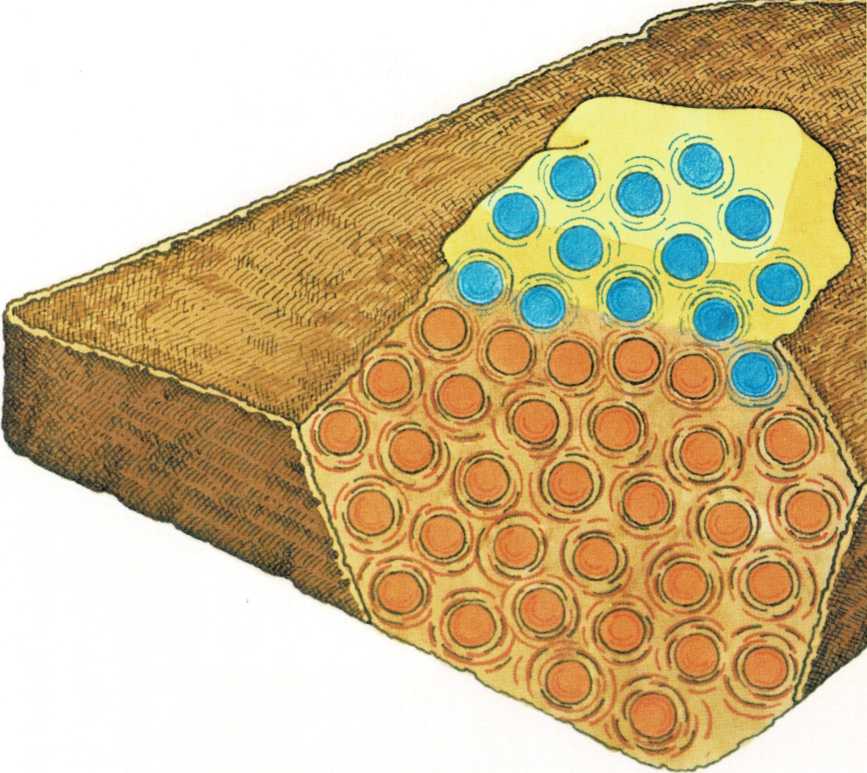
A slice of toast is a solid piece of bread. But the molecules in the
bread move—they jiggle and spin, even though they are held together.
As the bread is toasted, the heat
from the toaster makes the molecules speed up. The faster the molecules
move, the hotter the toast gets.
Cold butter is a solid, too—its molecules are moving, but they are
moving very slowly. When you spread the cold butter on the hot toast,
some of the slow-moving butter molecules touch the faster-moving
molecules in the toast.
The jiggling toast molecules bump against the slow-moving molecules in
the butter. That makes the butter molecules jiggle and spin faster. The
fast, jiggling motion spreads from molecule to molecule until all the
butter is soft and warm.
When you cook something in a pan, heat is conducted in the same way. The
heat from the stove speeds up the molecules of the pan and makes the pan
hot. Then the pan conducts heat to the food—its molecules bump against
the food molecules and speed them up until the food gets hot, too.